
CFZ selectively inhibits the chymotrypsin-like activity of the constitutive proteasome and the immunoproteasome. CFZ binds irreversibly to its target, resulting in sustained inhibition, which is in contrast to the reversible, boronate-based PIs, such as BTZ and MLN9708 –.
Ĭarfilzomib (CFZ) is a selective PI that is approved in the United States for the treatment of relapsed and refractory multiple myeloma (RRMM).
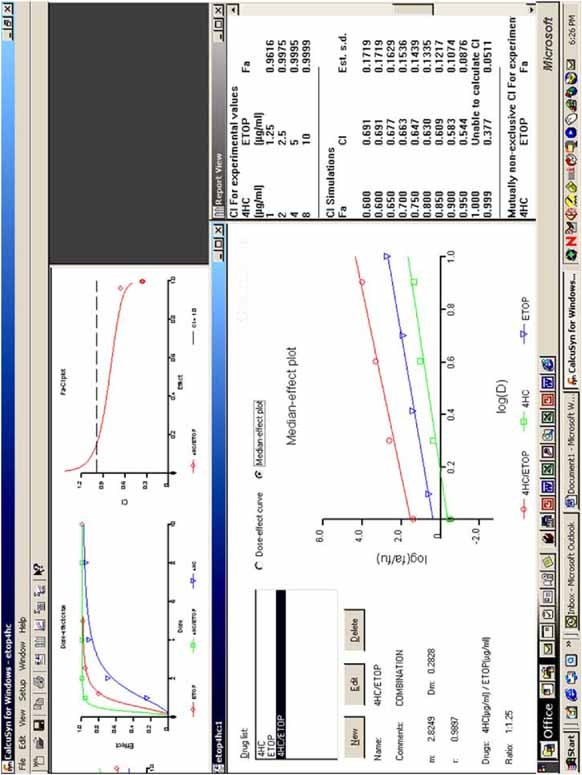
#CALCUSYN SYNERGY TRIAL#
In the setting of relapsed/refractory small cell lung cancer (SCLC), a clinical trial of BTZ reported limited single-agent activity. While BTZ showed potent in vitro activity in a wide range of non-small cell lung cancer (NSCLC) cell lines and demonstrated significant in vivo activity, clinical trials with BTZ monotherapy and in combination with chemotherapy or targeted agents in chemotherapy-naïve and previously-treated NSCLC patients yielded overall mixed results –.

Bortezomib (BTZ), the first-in-class Food and Drug Administration approved PI, has been investigated in preclinical models and in clinical trials as an anti-cancer therapeutic for lung cancer. There has been interest in proteasome inhibition as a therapeutic strategy in solid tumors, including lung cancer. Inhibition of the proteasome has proven to be an effective therapeutic strategy for multiple myeloma and mantle cell lymphoma. Other mechanisms by which proteasome inhibitors induce tumor cell apoptosis include phosphorylation and cleavage of the anti-apoptotic factor Bcl-2, stabilization of p53, interference with the unfolded protein response leading to endoplasmatic reticulum stress, and activation of TNF-related apoptosis-inducing ligand-induced apoptosis through increased death receptors DR4 and DR5 –. A dominant mechanism of action that contributes to the anti-tumor activity of proteasome inhibition is the down-regulation of proto-oncogenic nuclear factor kappa B (NF-кB) signaling through the blocking of inhibitory factor kappa B (I-кB) degradation inhibition of NF-кB signaling reduces expression of pro-inflammatory response genes and upregulates several cycle-dependent kinase inhibitors, promoting tumor cell apoptosis. Inhibition of the proteasome can induce disturbances in signal transduction, apoptosis regulation, cell cycle control, transcriptional regulation, and inflammation. Over the last several decades, proteasome inhibition has been extensively investigated as a selective anti-cancer strategy and validated in clinical trials using first and second generation proteasome inhibitors (PIs). ConclusionsĬFZ demonstrated anti-proliferative activity in lung cancer cell lines in vitro and resulted in a significant survival advantage in mice with SHP77 SCLC xenografts, supporting further pre-clinical and clinical investigations of CFZ in NSCLC and SCLC. In SHP77 flank xenograft tumors, CFZ monotherapy inhibited tumor growth and prolonged survival, while no additive or synergistic anti-tumor efficacy was observed for CFZ + cisplatin (CDDP). Western blot analysis of CFZ-treated H1993 and SHP77 cells showed cleavage of poly ADP ribose polymerase (PARP) and caspase-3, indicative of apoptosis, and induction of microtubule-associated protein-1 light chain-3B (LC3B), indicative of autophagy. CFZ had more variable effects in the SHP77 and DMS114 SCLC cell lines, with IC 50 values at 96 hours from <1 nM to 203 nM. CFZ had marked anti-proliferative activity in A549, H1993, H520, H460, and H1299 non-small cell lung cancer (NSCLC) cell lines, with IC 50 values after 96 hour exposure from <1.0 nM to 36 nM. ResultsĬFZ treatment inhibited both the constitutive proteasome and the immunoproteasome in lung cancer cell lines. MethodsĪ diverse panel of human lung cancer cell lines and a SHP77 small cell lung cancer xenograft model were used to investigate the anti-tumor activity of CFZ. The aim of this study was to investigate the activity of CFZ in lung cancer models. Phase 1B studies of CFZ reported signals of clinical activity in solid tumors, including small cell lung cancer (SCLC).
Carfilzomib (CFZ) is a proteasome inhibitor that selectively and irreversibly binds to its target and has been approved in the US for treatment of relapsed and refractory multiple myeloma.


 0 kommentar(er)
0 kommentar(er)
